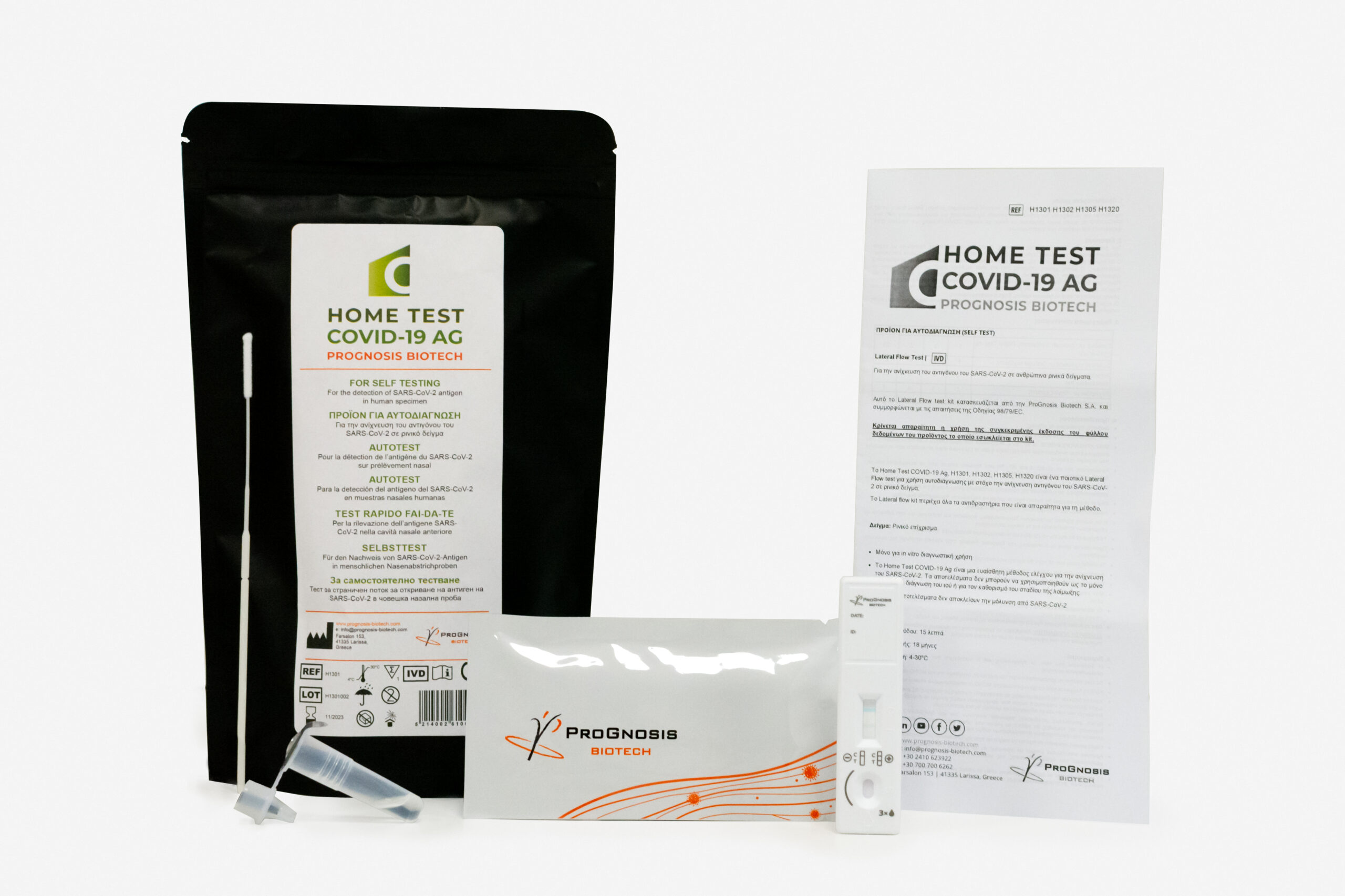
Specifications
LATERAL FLOW KIT CHARACTERISTICS:
■ Lateral flow assay (cassette format)
■ For private use/home use/self-testing against Covid-19
■ Clinical performance with 774 nasal specimens (prior confirmed with RT-PCR assay) showed: 96.85% sensitivity & 99.31% specificity
■ Rapid Test Ag 2019-nCov is confirmed to detect variants B.1.1.7 (Alpha), B.1.351 (Beta), B.1.429, B.1.427, B.1.2 , P1 (Gamma), P2, B.1.617.2 (Delta), B.1.617.3, C.37 (Lambda), P.3 (Theta), B.1.617.1 (Kappa), B.1.525 (Eta), B.1.526.1 B.1.526.2 (Iota) and the new variant B.1.1.529 (Omicron)
■ Visual interpretation of the results in 15 mins
■ Easy-to-use method
■ Using a minimally invasive nasal swab marked
■ CE marked by BQS (Notified Body 2854)
■ The kit contains all reagents required for the immunoassay method
■ Shelf Life: 18 months | Storage 4-30 °C