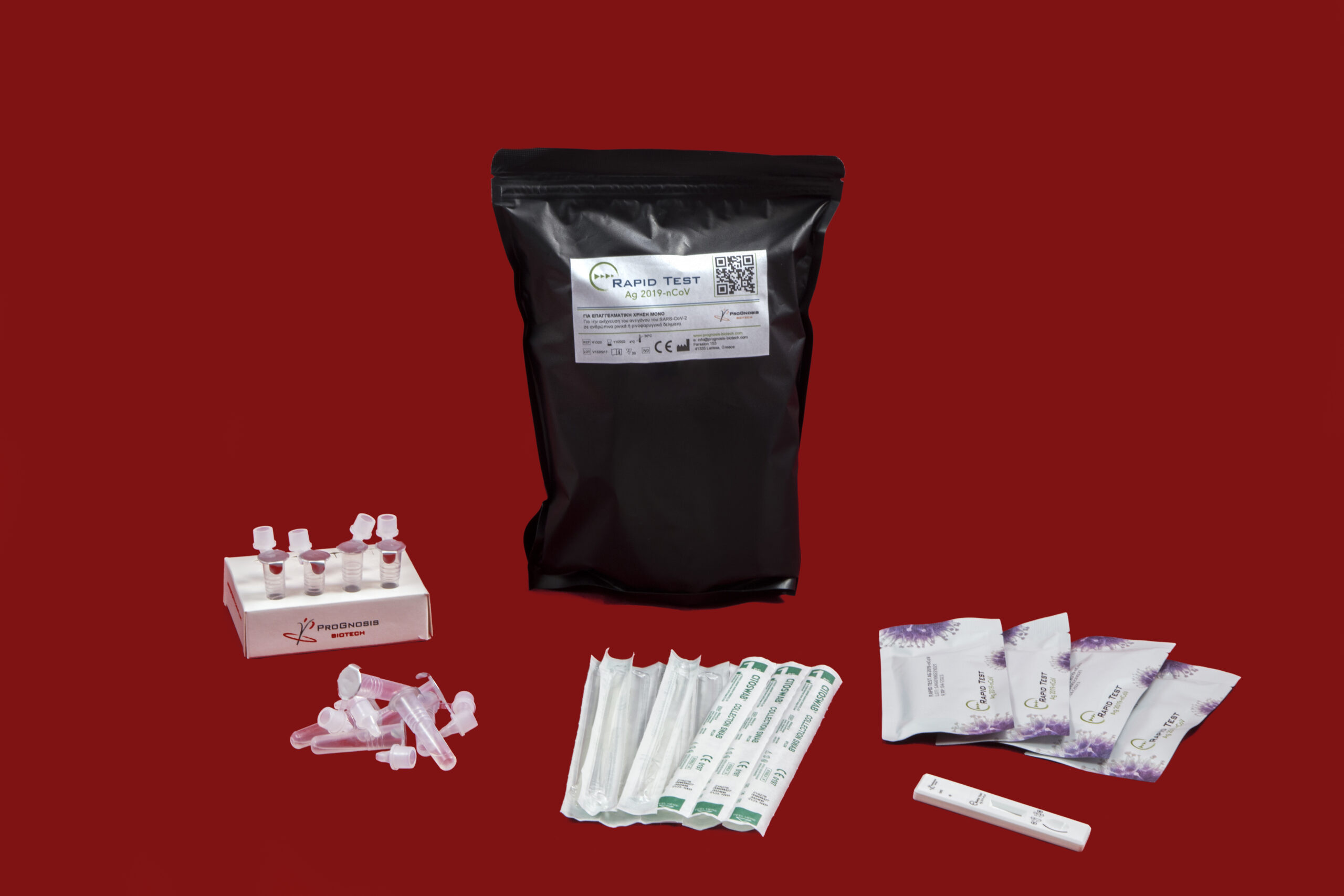
Specifications
LATERAL FLOW KIT CHARACTERISTICS:
■ Lateral flow in cassette format available in 1, 2, 5, 20 or 40 tests
■ Clinical Evaluation Study with 370 specimens (prior confirmed with RT-PCR assay) showed: 96.33% sensitivity & 99.62% specificity
■ Clinical performance with 528 nasal specimens (prior confirmed with RT-PCR assay) showed: 98.59% sensitivity & 99.74% specificity
■ The test provides the option for nasal swab sampling for less patient discomfort
■ Visual interpretation of the results in 15 mins
■ Simple method, no need for special equipment
■ Rapid Test Ag 2019-nCov is confirmed to detect variants B.1.1.7 (Alpha), B.1.351 (Beta), B.1.429, B.1.427, B.1.2 , P1 (Gamma), P2, B.1.617.2 (Delta), B.1.617.3, C.37 (Lambda), P.3 (Theta), B.1.617.1 (Kappa), B.1.525 (Eta), B.1.526.1 B.1.526.2 (Iota) and the new variant B.1.1.529 (Omicron)
■ Shelf Life: 24 months | Storage 4-30 oC
■ The test has received the CE-IVD
■ Test should only be conducted by medical personnel